Ethylene Oxide Sterilization Iso - Ethylene Oxide Sterilization Validation Ppt Video Online Download
All sterilizers are equipped to meet the strict requirements of ANSIAAMIISO 11135. Process of obtaining and documenting evidence that installed equipment operates within predetermined limits when used in accordance with its operational procedures The difference in these two.

Ethylene Oxide Sterilization Validation Ppt Video Online Download
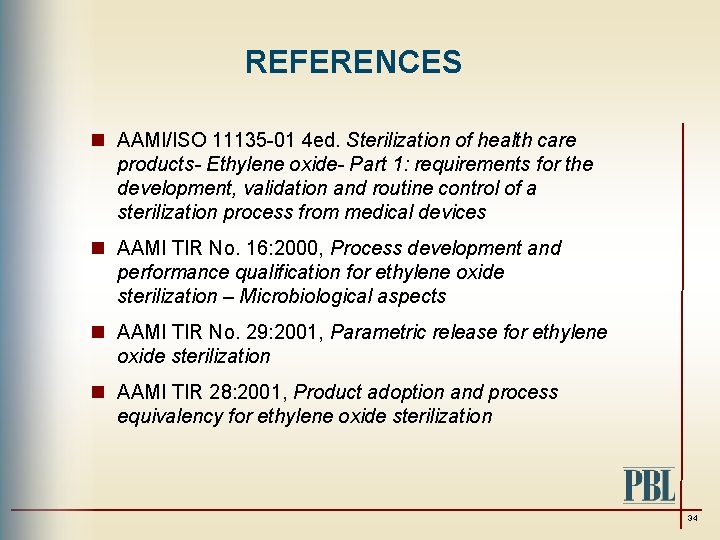
ISOAWI 11135 Sterilization of health-care products Ethylene oxide Requirements for the development validation and routine control of a sterilization process for medical devices.

Ethylene oxide sterilization iso. ISO 11135 was updated in July 2014 and contains. ISO 11135-12014 the international standard for ethylene oxide EO sterilization validation provides a slightly different definition for an OQ. Validation and routine Control of a Sterilization Process for Medical Devices Ethylene Oxide EO Sterilization and Validation ISO 10993-72008 R 2012 Biological evaluation of medical devices - Part 7.
Requirements for development validation and routine control of a sterilization process for medical devices ANSIAAMIISO 11138-22006R2010 Sterilization of. AAMI Arlington VA 2009 27 ASNZS 4187 2 Reprocessing of reusable medical devices in health service organizations 28. ISO 11135 Sterilization of health care products Ethylene oxide Requirements for development validation and routine control of a sterilization process for medical devices.
Sterilization of health care products - Biological indicators - Part 2. ISO 111352014 Sterilization of medical devices Requirements for the development. Biological indicators for ethylene oxide sterilization processes ISO 11138-22006 - SS-EN ISO 11138-22006This part of ISO 11138 provides specific requirements for test organisms suspensions inoculated carriers biological indicators and test methods intended f.
Overview of Ethylene Oxide EO or EtO Residuals. Microbiological aspects of ethylene oxide sterilization. Ethylene Oxide residues.
This is now the only applicable Standard. The gas is an alkaline agent that infiltrates packaged medical devices to kill microorganisms and thus achieve sterilization. For ethylene oxide sterilization two voluntary consensus standards ANSI AAMI ISO 111352014 and ANSI AAMI ISO 10993-72008R2012 describe how to develop validate and control ethylene oxide.
The overkill method AAMIISO 11135 Method C is most commonly used when performing an EtO sterilization validation. Ethylene Oxide EO Sterilization Process Components. ANSIAAMIISO 11135 2007 Medical devices Validation and routine control of ethylene oxide sterilization 2 ST23 Guideline for Industrial Ethylene Oxide Sterilization of Medical Devices Process Design Validation Routine Sterilization and Contract Sterilization 1995 1988.
Facility meets all Clean Air Requirements including Ethylene Oxide Standards for Sterilization Facilities. EtO Sterilization 2019-06-20T235811-0700 One of the most popular methods of sterilization of medical devices is through exposure to Ethylene Oxide gas EtOEO. This standard has been revised by ISO 10993-72008 Abstract Specifies allowable limits for residual ethylene oxide EO and ethylene chlorohydrin ECH in individual EO-sterilized medical devices and procedures for the measurement of EO and ECH.
The overkill method is based on demonstrating that the sterilization of a microbial challenge biological indicator exceeds the. As medical devices must pass specific sterility standards before being used on patients ethylene oxide sterilization is a simple effective and common heat sterilization. Sterilizing medical devices with ethylene oxide EO is a common practice primarily due to its extensive material compatibility.
O Changes to Requirements and a o Significant amount of new guidance o As of July 2017. United States Pharmacopeia 31st ed 2008. The main side effect of using EO as a sterilization agent is that it can leave a residue on the devices being processed.
Ethylene oxide Part 1. Sterilization of health care products Ethylene oxide Part 1. Requirements for development validation and routine control of a sterilization process for medical devices 9599 ISOTC 198.
Ethylene oxide EO is a gas used to sterilize medical devices particularly devices that are unable to be sterilized with traditional high heat. 1232 Requalification of a sterilization process carried. AAMI Arlington VA 2009 26 AAMI TIR28 Product adoption and process equivalence for ethylene oxide sterilization.
This international standard outlines the requirement for the validation of an EO process to ensure that product processed through the validated process meets the. Ethylene oxide sterilization residuals 1 Scope This part of ISO 10993 specifies allowable limits for residual ethylene oxide EO and ethylene chlorohydrin ECH in individual EO-sterilized medical devices procedures for the measurement of EO and ECH and methods for determining compliance so that devices may be released. Ethylene oxide sterilization residuals EO Residuals.

Iso 10993 7 Biological Evaluation Of Medical Devices Ethylene Oxide

Onorm En Iso 11135 2015 Sterilization Of Health Care Products Ethylene Oxide Requirements For The Development Validation And Routine Control Of A Sterilization Process For Medical Devices Iso 11135 2014 Austrian Standard

Iso 11135 1 2007 Sterilization Of Health Care Products Ethylene Oxide Part 1 Requirements For Development Validation And Routine Control Of A Sterilization Process For Medical Devices

Iso 10993 7 1995 Biological Evaluation Of Medical Devices Part 7 Ethylene Oxide Sterilization Residuals Iso Tc 194 Wg 11 Amazon Com Books

Iso 7000 2501 Sterilized Using Ethylene Oxide

Une En Iso 11135 2015 Sterilization Of Health Care Products Ethylene Oxide Requirements For The Development Validation And Routine Control Of A Sterilization Process For Medical Devices Iso 11135 2014 European Standards

Iso 11135 1 2007 Sterilization Of Health Care Products Ethylene Oxide

Ethylene Oxide Sterilization Validation Pacific Bio Labs Inc

New Guidance On Parametric Release For Ethylene Oxide Sterilization

Ethylene Oxide Eo Sterilization Validation Procedure

Iso 11135 2014 Sterilization Of Health Care Products Ethylene Oxide Requirements For The Development Validation And Routine Control Of A Sterilization Process For Medical Devices International Organization For Standardization 9789267107011
Ethylene Oxide Sterilization Validation Pacific Bio Labs Inc

En Iso 11135 2014 A1 2019 Sterilization Of Health Care Products Ethylene Oxide Requirements

En Iso 11135 2014 A1 2019 Sterilization Of Health Care Products Ethylene Oxide Requirements

Iso 10993 7 European Standards

Eo Sterilization Residual Testing Services Dye Penetration Texas

Iso 10993 7 2008 Biological Evaluation Of Medical Devices Part 7 Ethylene Oxide Sterilization Residuals
